Measuring surface energy for successful bonding
- Measuring surface energy to choose the right industrial tape or adhesive
- Classification of materials by surface energy
- Basic concepts and measurement methods of surface tension
Quote
Close
You request has been sent.
We promise to respond to you in detail within 24 hours.
In the meantime, feel free to browse our other products.
Why measure the surface tension before bonding ?
Surface energy, also called surface tension, is a good indicator to characterize the suitability of your materials to be bonded with an industrial tape or adhesive.
This concept highlights the way in which the molecules of a fluid or a material are attracted to each other and to the molecules of another environment.
Low surface energy materials, such as Teflon, PTFE or polyethylene, are difficult to bond and require the use of appropriate adhesive tapes or glues and/or specific treatment to increase their surface energy.
This is why surface tension is a fundamental concept in the field of technical tapes and adhesives. It is sometimes necessary to measure it in order to achieve a difficult bond, especially for a permanent assembly.
Little experiment to understand surface tension
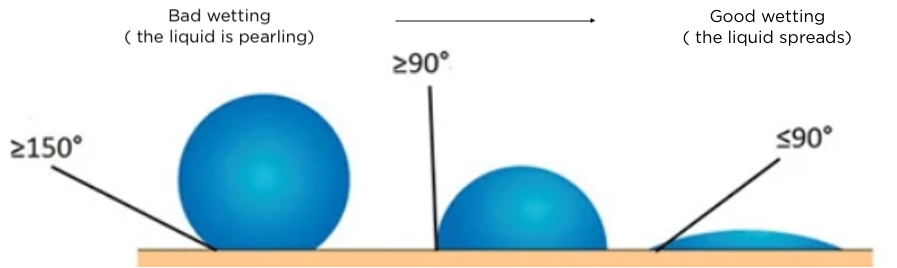
To illustrate the surface energy of a material, we observe the behaviour of a water drop on this substrate. The larger the contact area between the drop and the material, the higher the surface energy. The ability of a liquid to spread on the surface of a solid is called "wetting".
High surface energy materials are easier to wet than low surface energy materials.

In the photo above, the left side of the stainless steel substrate has a low surface energy due to the addition of a non-stick treatment. The right hand side, untreated, has a high surface energy and the water drop spreads. This part is therefore more likely to be effectively bonded or glued.
The ease with which an adhesive wets the surface of a material therefore depends on the surface energy of the material. This is expressed in units of energy per surface. Its unit of measurement is usually expressed in "dyne" (1 dyne/cm = 1 mJ/m) or in mN/m.
We will come back to the notion of wetting/wettability in detail later.
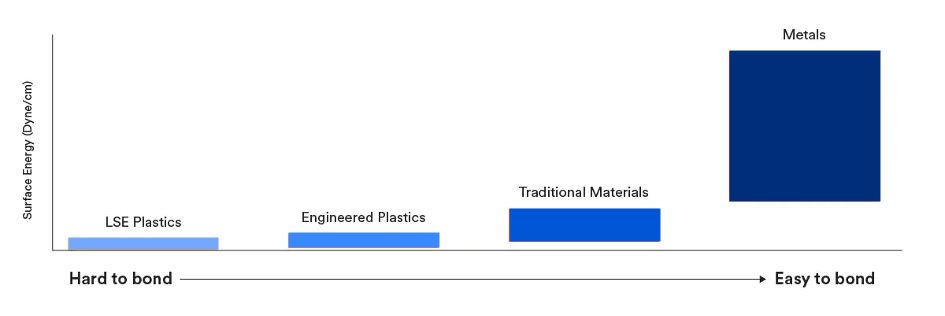
The 3 categories of surface energy of materials
LOW SURFACE ENERGIES(< 36 dynes/cm)* |
MEDIUM SURFACE ENERGIES(36 to 300 dynes/cm)* |
HIGH SURFACE ENERGIES(> 300 dynes/cm)* |
|---|---|---|
|
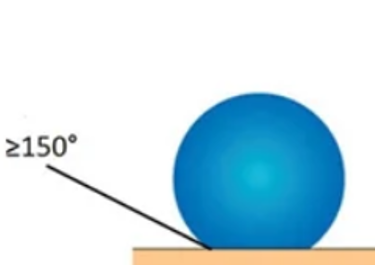
Molecules on the surface of low surface energy materials are very little attracted to other molecules, especially adhesive molecules. Low surface energy materials are therefore very difficult to bond. |
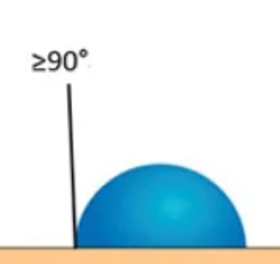
Medium surface energy is quite relative and lies between the spreading wetting film and the spherical drop. | The molecules on the surface are very strongly attracted to the molecules around it. Materials with high surface energy, most metals, are easy to wet and therefore easy to bond. |
 |
 |
 |
Be aware that "non-stick" release agents such as polytetrafluoroethylene (PTFE) can be problematic for bonding. |
They are fairly easy to bond/glue, but there are specific factors to consider for each one, including surface preparation and cleanliness.
|
Adhesives and tapes are particularly suitable solutions for this type of material. They can even provide additional qualities to a bonding/assembly, such as thermal insulation, electrical insulation, filling (jointing, sealing). |
* generally accepted theoretical reference values
Classification of materials by surface energy
Table updated regularly from our laboratory tests.
| TYPE OF SURFACE ENERGY | NATURE |
ABREVIATION OR SYMBOL |
MATERIAL | TRADE NAMES | VALUE (DYNE/CM) |
|---|---|---|---|---|---|
| High surface energy | Metals | Cu | Copper | 1103 | |
| Al | Aluminium | 840 | |||
| Zn | Zinc | 753 | |||
| Inox | Stainless steel | 700 - 1100 | |||
| Sn | Tin | 526 | |||
| Pb | Lead | 458 | |||
|
Medium surface energy
Examples of suitable adhesive tapes:
|
Traditional materials | - | Enameled or glazed ceramics | 250 - 500 | |
| - | Unglazed ceramics | 200 - 400 | |||
| - | Glass | 200 - 300 | |||
| - | Cement | 70 | |||
| - | Wood | 40 - 50 | |||
| - | Synthetic/polyester fabric | 45 | |||
|
Engeneering plastics |
PI | Polyimide | Kapton | 50 | |
| PF | Phenoplast/Phenol-formaldehyde resins | 47 | |||
| PA | Polyamide | Nylon, Rilsan | 46 | ||
| - | Alkyd enamel | 45 | |||
| PET | PolyEthylene Terephtalate | Alkyd | 43 | ||
| EP | Epoxy paint | 43 | |||
| PUR | Polyurethane | 43 | |||
| ABS | Acrylonitrile butadiene styrene | Cycolac, Abson | 42 | ||
| PC | Polycarbonate | Lexan, Makrolon, Arla | 42 | ||
| PVCr | Rigid PolyVinyl Chloride | Forex, Komacel | 39 | ||
| PPE | PolyPhenyl Ether | 38 | |||
| PPO | Polyphenylene oxide | Noryl | 38 | ||
| PMMA | Poly(Methyl MethAcrylate) | Plexiglass, Acrylex, Polivar | 38 | ||
|
Low surface energy
Examples of suitable ahdesive tapes:
Examples of suitable glues :
|
LSE plastics | PS | Polystyrene | 36 | |
| POM | Polyacetal/Polyoxymethylene Acetal | Derlin | 36 | ||
| EVA | Ethylene-Vinyl Acetate | 33 | |||
| PE | Polyethylene | 31 | |||
| PP | Polypropylene | Akylux, Ethylux | 29 | ||
| PVF | PolyVinyl Fluoride | Tedlar | 28 | ||
| PTFE | Polytetrafluoroethylene | Teflon, Dyneon | 18 |
Be careful with some substrates :
- Soft PVC: migration of plasticizers
- Metals and alloys: possible presence of an oxide layer
- Epoxy powder paints: surface tension agents and other additives modifying the characteristics of the paint to optimize its resistance to environmental constraints (gloss, scratch resistance, temperature...)
- ...
Always check if your surface to be bonded has been covered with a special coating (paint, epoxy paint, varnish, non-stick release agents...). On these surfaces, the material to be bonded is the coating, not the material underneath !
For each application, ask for technical expertise to find the right single-sided, double-sided tape or adhesive.
Define surface energy: Fundamentals
By measuring the surface tension of a solid material and the surface tension of an adhesive mass, it will be possible to choose the appropriate adhesive tape or glue and/or treat the surface to be bonded in order to obtain the desired wettability and optimize your bonding.
Surface energy of solids: the wetting
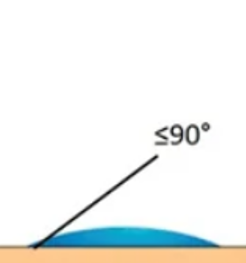
As seen previously, when a drop of fluid is deposited on a solid, it will spread more or less well on its surface by forming an angle called "contact angle" between the fluid/air interface and the solid surface. This phenomenon is called "wetting".
- Low surface tension of the liquid = Wetting liquid
- High surface energy of the substrate = Easy to wet substrate
Surface tension of liquids
Have you ever observed some of these phenomena in your daily life? Insects walking on water, dew beading on the petals of a flower, sugar soaking up coffee, or a water drop hanging without falling? These fun facts are all the effect of the surface tension of liquids.
It is a physico-chemical phenomenon linked to the molecular interactions of a fluid: that is to say the way in which its molecules are attracted to each other on the one hand, and to the molecules of another material on the other.
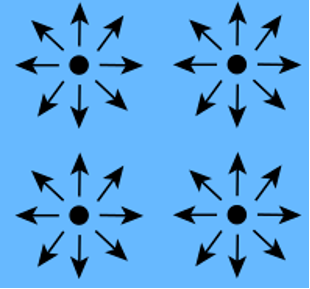
Molecules within a fluid exert forces of attraction or repulsion between them:
- Van der Waals force (attraction)
- Electrostatic force (attraction or repulsion, hydrogen bond)
These molecules are always positionned in the lowest energy configuration within the same fluid in order to reach an energy balance.
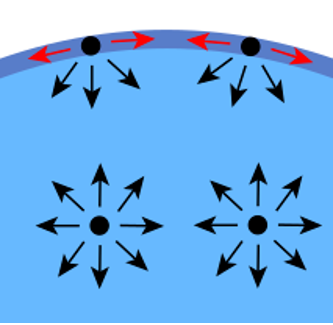
In a system involving several environments, the molecular interactions are unbalanced: the molecules located at the interface interact with those of the other environment, but those located within the matter interact only with their fellow molecules.
The system naturally tends towards and equilibrium corresponding to the configuration of the lowest energy. It then modifies its geometry to decrease the area of the interface with the other environment. The force that maintains the system in this configuration is the surface tension.
Surface tension is a good indicator of the ability of a fluid to wet and therefore of the ability of an adhesive mass to adhere to a given surface.
Methods to measure surface tension
Here are two examples of methods frequently used to measure surface energy. Our application engineer can guide you in your measurements, but also carry out certain tests in our internal laboratory, in order to guide you in the choice of your products and the preparation of your surfaces to be bonded.
Sessile drop method
Using a goniometer, composed of a motorized syringe connected to a data processing software, we are able to deposite a drop of liquid on a solid substrate under study. A camera collects and records the images of the experiment. We observe the shape of the drop, the contact angle, and by cross-referencing these data with the determined surface tension of the fluid, we are able to calculate the surface energy of the substrate.
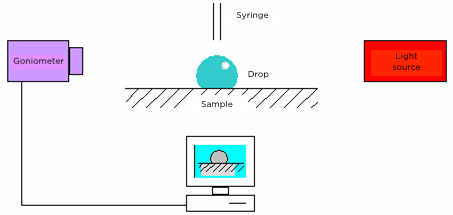
Note: Water is generally used for the basic surface energy measurement. It is easy to purify and has a low viscosity, so surface equilibrium can be reached in less than a minute. Other fluids, such as certain solvents or oils, can be used to refine the measurements later.
Test inks method
The test ink method of measuring surface energy is a simple and fast process, requiring less equi^ment than other methods. It is particularly used to measure the surface energy of common materials, such as plastic, glass, recycled or composite materials. The tester has several bottles of ink, corresponding to different ranges of surface energy values.
- After cleaning and possibly pre-treating the surface to be tested, the ink corresponding to the highest surface energy is applied with a brush integrated in the bottle.
- If the applied ink is stable for 2 seconds, the surface is easily wettable and thus adherable.
- If the ink beads on the substrate, the test is continued with the next lower value and so on, until the applied ink is stable.
The surface tension of the material is equal to the value of the last ink tested that showed a bad wetting for at least 2 seconds.
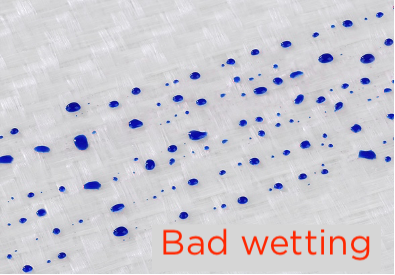

Note: The test ink method requires a critical approach to the measurement results. The values obtained are within a relative range of results. In all cases, the inks used must comply with DIN 53364 or ISO 8296.
Obstacles, contaminations and treatments
Contamination of the surface to be bonded can cause:
- A loss of tack (instant adhesion)
- A decrease in wetting
- Reduced adhesive/substrat contact
- Reduced adhesive strength
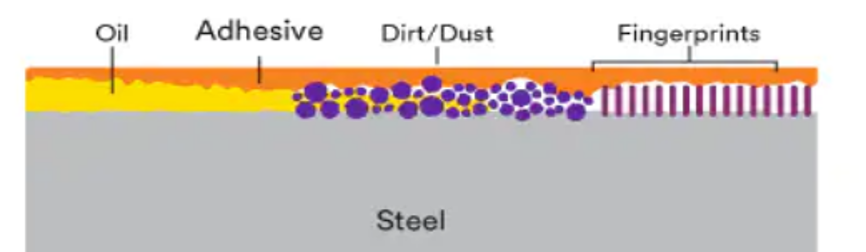
These obstacles reduce the adhesion surface, weaken the bonding and increase the chances of the tape tearing. In order to prevent this kind of inconvenience, we remind you that a degreasing according to precise methods is essential before any gluing/assembly.
The application of a bonding primer can also be used to increase the surface energy of a substrate, especially for your difficult bonding (low surface energy materials).
See our page on surface preparation before applying an adhesive solution for more details and technical tips.